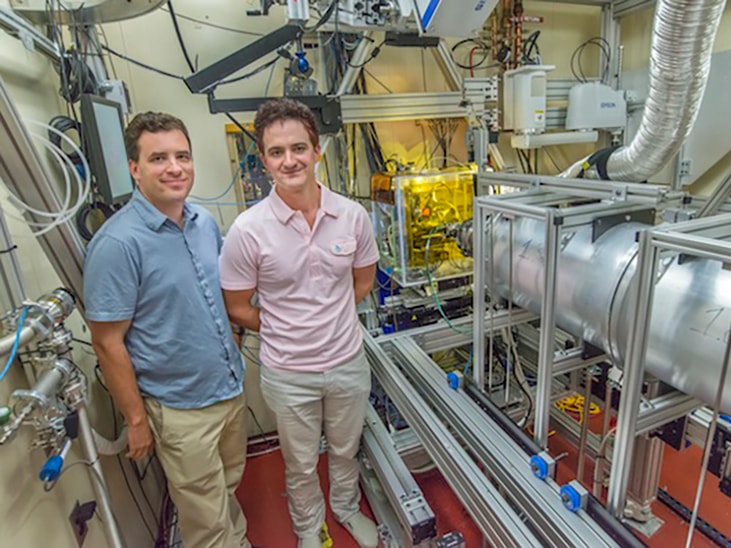
Berkeley Lab awarded $8m for research in the hydrogen and fuel cell sector
With commitments from leading car and stationary-power manufacturers to hydrogen and fuel cell technologies and the first ever fuel cell electric vehicle to go on sale later this year, interest is once again swelling in this carbon-free technology.
Now, thanks to several new projects from the US Department of Energy’s (DOE) Fuel Cell Technologies Office, scientists from Lawrence Berkeley National Laboratory (Berkeley Lab) will have an important role in accelerating innovation and commercialisation of hydrogen and fuel cell technologies.
Berkeley Lab has been awarded $8m for two new DOE research efforts, one to find new materials for hydrogen storage and another for optimising fuel-cell performance and durability.
In addition, Berkeley Lab is leading a range of other hydrogen and fuel cell research projects aimed at developing next-generation fuel cell and related energy-conversion technologies.
... to continue reading you must be subscribed