CAIRE Inc. acquires Spirosure, broadens product portfolio
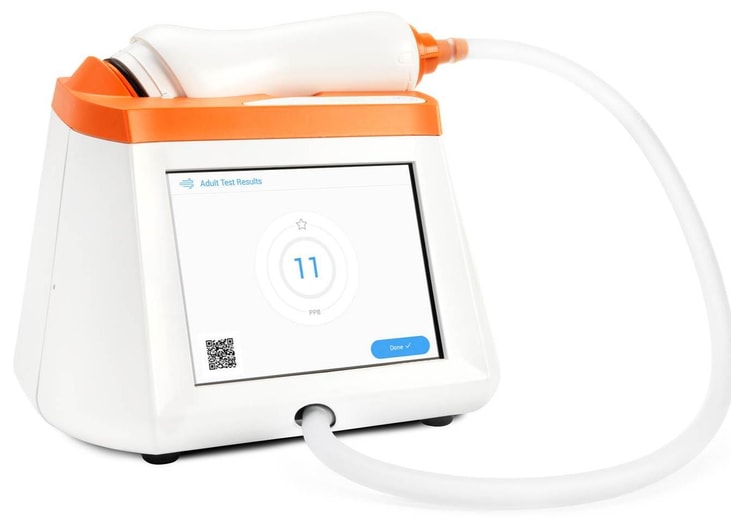
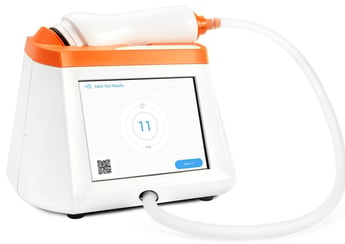
CAIRE Inc. has acquired Spirosure, Inc. to expand CAIRE’s portfolio into the diagnostic segment of respiratory care.
Spirosure is a California-based developer and manufacturer of an innovative technology for measuring fractional exhaled nitric oxide, which is a key indicator of allergic inflammation in asthmatic patients.
Spirosure will operate as a division of Georgia, US-based CAIRE to be known as CAIRE Diagnostics Inc.
... to continue reading you must be subscribed