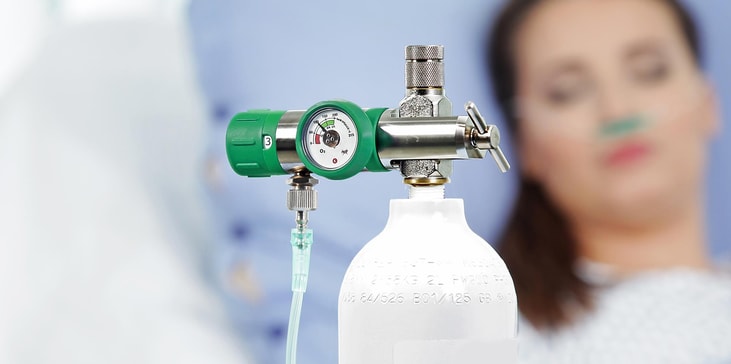
GCE assistance for Covid infection management
Treatment for severe cases of Covid-19 often involves supplemental oxygen therapy or ventilation. Consequently, equipment such as regulators, flowmeters and cylinder valves have been in peak demand across hospital and homecare settings worldwide.
In the course of delivering such care, medical devices and instruments will come into contact with patients infected with coronavirus. In order to prevent potential patient-to-patient transfer of Covid, reusable medical devices must be sterilized or thoroughly disinfected between uses.
Understandably, medical professionals are keen to ensure that they are following effective cleaning and disinfecting regimes and actively managing the risk of infection. In the United States, such advice is stipulated by the US Center for Disease Control & Prevention (CDC) which has a list of EPA-registered disinfectants that meet the criteria for Covid-19.
... to continue reading you must be subscribed