Life sciences company invests in cryogenics production line
Novasep, supplier of services and technologies for the life sciences industry, has announced a €4m ($4.8m) investment to expand cGMP (current good manufacturing practice) capacity for clinical and commercial supply of active pharmaceutical ingredients (APIs) at its Chasse-sur- Rhône facility.
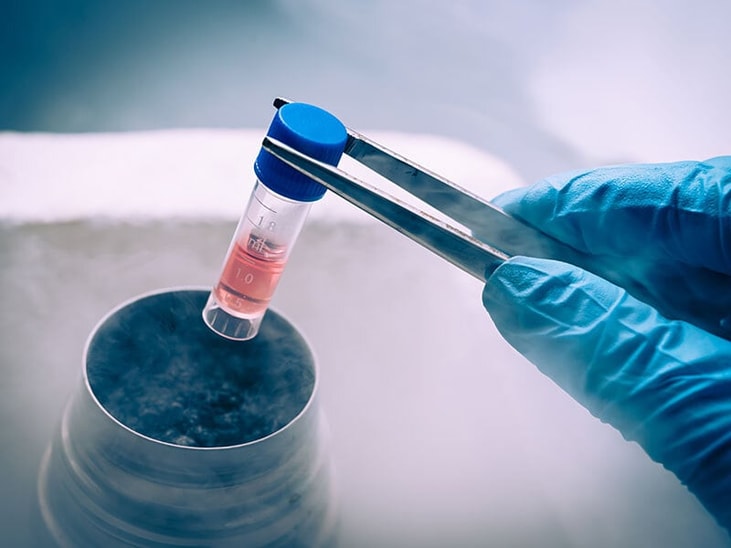
The investment includes the installation of a new cGMP cryogenics production line, capable of operating at temperatures as low as -80℃. There will also be expanded cGMP pilot-plant capabilities with the addition of a new stream comprising a 400L Hastelloy reactor, filter drier and clean room. This will add flexible small-scale manufacturing capacity and reinforce the site’s capability to handle both clinical development needs and low volume APIs.
Jean-Pierre Pilleux, General Manager of the Chasse-sur-Rhône site said, “By increasing cryogenics capacity at the current Chasse-sur-Rhône facility, we strengthen Novasep’s small and large-scale volume offering for low temperature manufacturing.” He continued, “It will give us more flexibility and allow us to address the increasing market demand for these types of capabilities”.
As a contract development and manufacturing organisation (CDMO), Novasep has a 30-year track-record in the scale up, validation and commercial supply of low-temperature chemistry under cGMP. These new facilities will enable Novasep to handle highly reactive compounds such as organometallic reagents and improve the selectivity of reactions.
... to continue reading you must be subscribed