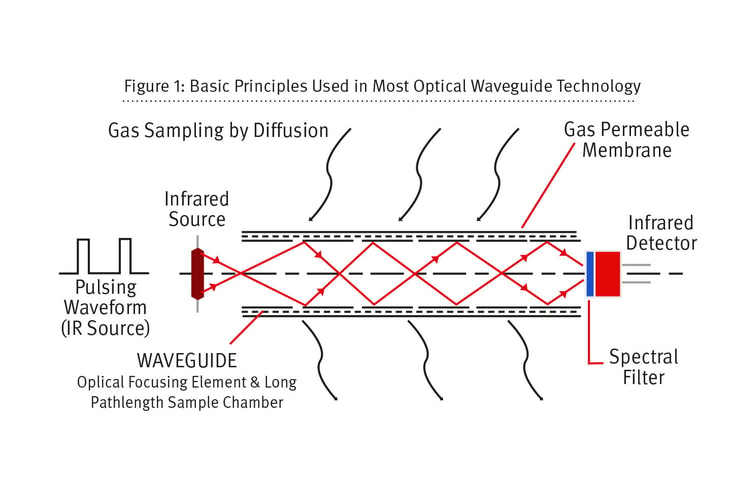
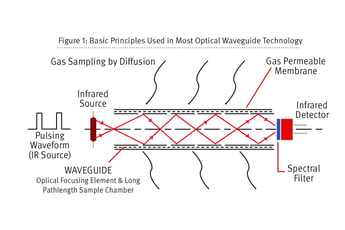
Living with CO2 sensors: Understanding leak detection and the responsibility of calibration
Now that carbon dioxide (CO2) monitors are legislation in most countries and laws are in place in all states in the US since a while back, it is important to know how to live with them and how to make a ‘calibration’ or check-up of the functionality of the sensors.
It is important to know that a calibration adjustment doesn’t have to be made to a sensor that shows the right value – you don’t change the time on your watch if it is showing the right time.
This is a requirement in most places, but we find that it is neglected and not done according to the authorities’ directives. We believe that just as with elevators and such equipment, there should be a sticker on the sensor that indicates that it has been done and the time when it should be done again.
... to continue reading you must be subscribed