Oxygen: New Oxygen 98 monograph to be considered
The European Directorate for the Quality of Medicine and Healthcare (EDQM) has revealed that it is launching an extraordinary public consultation on how best to include oxygen at 98% purity obtained via two-stage concentrators in European Pharmacopoeia.
The development is largely driven by the heightened need for medical oxygen during the current coronavirus pandemic, as widely reported by gasworld.
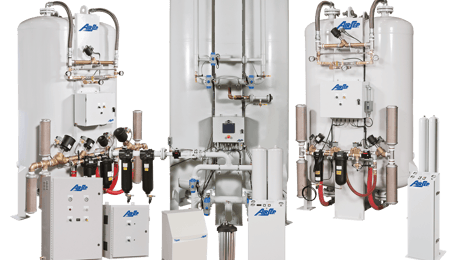
However, it is also as a result of the advances in technology since the last monograph was published, particularly the rise of double-stage pressure swing adsorption (PSA) oxygen generators.
... to continue reading you must be subscribed