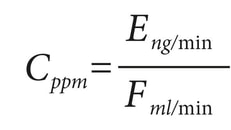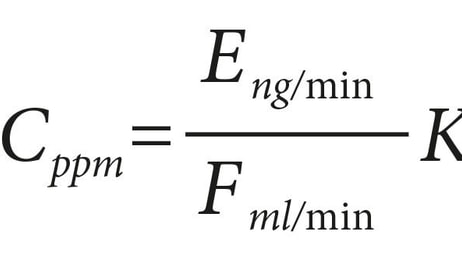Surviving your next FDA inspection
As FDA inspection guidelines and expectations undergo significant evolution, Ron Ball explains how to prepare for all eventualities.
Over the last 3-4 years the FDA has been targeting its inspectional resources to establish the infrastructure needed to inspect the growing number of foreign drug manufacturing facilities who now ship products to the US. The agency is now waking up to the fact that it has fallen far behind schedule in inspecting drug manufacturing sites in the US, and is just now beginning to re-focus its attention on the backlog of audits at US drug manufacturers. Many US medical gas firms have not seen an FDA inspector in their facility for years, but that is about to change.
This article will examine some do’s and don’ts when preparing for your next FDA inspection.
... to continue reading you must be subscribed