MOCON launches non-invasive oxygen analyser capability for pharmaceutical applications
Minneapolis-based MOCON, Inc., one of the world’s leading manufacturers of permeation instrumentation, has launched a new capability for its OpTech® – O2 system.
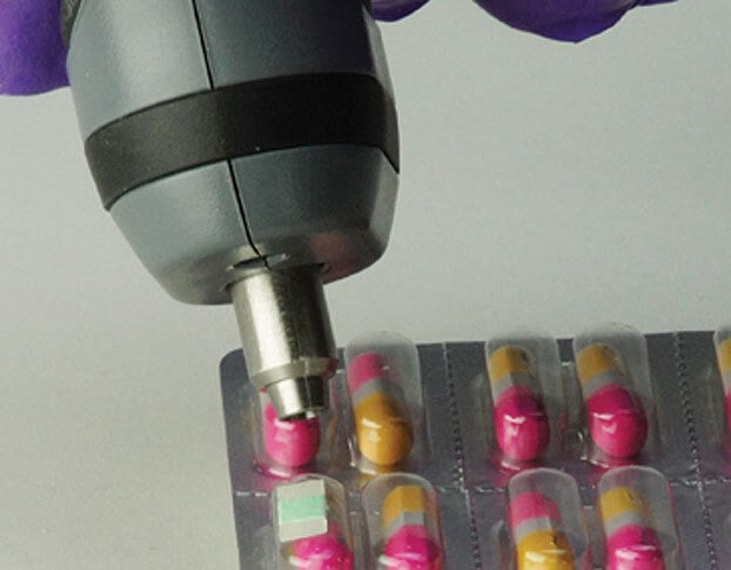
Non-invasive, non-destructive thermoformed blister package testing is now possible due to the development of an optical sensor that will ‘fluoresce’ or give off light directly related to the amount of oxygen present.
OpTech® – O2 technology is ideal for determining oxygen headspace and ingress in flexible and rigid packaging.
“Some blister packaging applications depend on gas flushing to enhance the product’s shelf life. However, modified atmosphere can be compromised by poor packaging seals, material breakdown, weak barrier properties, pinholes. This is why using a precise and sensitive package performance test method is so important—especially for drug packaging,” said Ed Emerson, Manager of Healthcare Business Development at MOCON.
... to continue reading you must be subscribed