UArizona invention could help coronavirus patients breathe easier
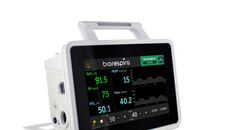
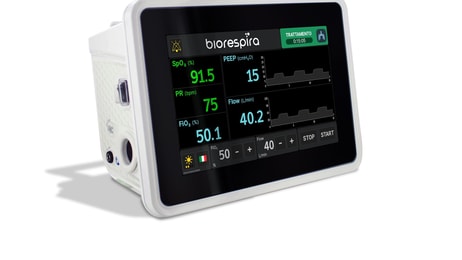
Researchers at the University of Arizona College of Medicine – Tucson have invented a new respiratory-assist device (RAD) that provides fast, safe relief to those experiencing difficulty breathing amid coronavirus (Covid-19).
One of the major complications of Covid-19 is that it causes inflammation in the respiratory tract and lungs that can lead to life-threatening pneumonia.
In such cases, the fast and safe application of a RAD can make the difference between life and death.
The small-scale, low-pressure heliox rebreathing system simultaneously removes carbon dioxide while appropriately adjusting for humidity as it recirculates gases in a closed system.
... to continue reading you must be subscribed